Globalization in Egypt in a Historical Context: Berthollet and the Egyptian Natron
Journal: Journal of Globalization Studies. Volume 11, Number 1 / May 2020
DOI: https://doi.org/10.30884/jogs/2020.01.08
In 1798, Napoleon Bonaparte conquered Egypt with armed forces numbering 54,000 warriors, which was accompanied by a party of about 150 civilian intellectuals – researchers, analysts, mathematicians, engineers, astronomers, doctors, chemists and other specialists – whose objective was to record the culture of Egypt. The result was an extensive series of writings and publications known as the Description de l'Égypte. Napoleon selected the famous French chemist Claude Louis Berthollet (1748–1822) to go with him as a logical advisor in the foremost of his far-flung campaigns. Once in Egypt and while visiting the Natron Lakes, a series of saltwater lakes carved from limestone, Berthollet made an observation that led to an important discovery. While exploring the shore of the lakes, Berthollet found deposits of Na2CO3, a result he found was surprising. Why did Berthollet find this result surprising, and therefore the way did it contribute to an important discovery?1 In the present paper, I answer this question by showing the extent to which Berthollet's research on the Natron salts of Egypt is highly valuable for theories of affinity in chemistry.
Keywords: history of science, Egypt, Claude Louis Berthollet, the Napoleonic scientific expedition, the Natron from Egypt, theories of affinity, Chemistry.
Hamed A. Ead, Cairo University more
Introduction
In the introduction to his interesting article comparing globalization in historical retrospective and within the world-system analysis, Thomas Hall (2014) after giving a full background and underlying theoretical and substantive debates about globalization, and world-systems analysis, concludes that ‘the entire issue, is not intended as a final word, or even current word. Rather, it is an invitation to scholars in many disciplines to contribute to these discussions’ (Ibid.: 3). In theory, the concept of globalization is explained by its name: people from different countries with different cultural backgrounds are connected with each other economically, socially and politically so that they form a standard homogenous culture and thus become globalized. Globalization weakens the restrictions of national boundaries. Giddens states, ‘globalization is about the intensification of worldwide social relations which link distant localities in such a way that local happenings are shaped by events occurring many miles away and vice versa’ (Giddens 1990: 64). According to Alexey I. Andreev and his coauthors, ‘the “age” of global world does not equal to the “age” of human history from the perspective of Big History which is one of the most groundbreaking fields in historical research viewing the integrated history of Space, Earth, life and humanity on large-scale time spans using a multidisciplinary approach’(Andreev, Ilyin, and Zinkina 2015: 250).
When Napoleon Bonaparte landed on Egypt's Mediterranean coast near Alexandria on July 1, 1798, he wanted to colonize Egypt and thus, to usurp control from the powerful Mamluks who had ‘exclusively favored English commerce’ to the detriment of the French trade interests (Matteson 1910). Aside from these economic interests and following the ideas of the French Enlightenment to liberate people from their suppressive regimes, Napoleon seems to have believed that the local population would be receptive to the French (Johnston 2003). He was horribly mistaken: the Egyptian population did not welcome the French and consequently, Napoleon's objectives to establish a French government and implement French systems failed. Three years later in March 1801, the British and Ottoman forces defeated the French in Egypt and ended this episode of the Egyptian history. For over 200 years, the French occupation has been a source of discussion and debate among scholars and laypeople as well. Most of the discussion and contentious debate has centered on the degree of impact or influence the French occupation had on the subsequent history of Egypt. ‘Globalization is no less than a development of the Enlightenment project of modern social theory and practice, the urge to control the world so as to explain it, to explain the world so as to promote change, and to change the world so as to control it’ (Deacon 2005: 34). So, the French occupation of Egypt between 1798 and 1801 was the first colonial conquest which endeavored to bring the Enlightenment to the Orient. The invasion was justified exclusively by the assumed superiority of the Western value system, ‘liberating’ the Orient from the yoke of barbaric despots.1
2. The Egyptian Civilization
The Egyptian civilization is one of the most prominent civilizations around the world, most likely dating back to 3400 BC, that is, to the ancient times. This profoundly created civilization kept going for more than 3000 years, during which it spread broadly. In fact, some archeologists claim that they trace the signs of the Egyptian origin in other civilizations. However, it may be universally agreed that Egyptian craftsmen made their way to the rest of the world. Although everybody recognizes that Egypt is the place of origin of the practices and chemical applications, tragically, there is little remaining within the early records that could clarify those statements. Our information on the early advancement of chemical craftsmanship depends on the revelation of items made of tin and bronze up to around 3000 BC. There are primitive expressions that provide information related to chemistry, such as those of metallurgy, glassmaking, coloring and treating, many of which reached an astonishingly high level of perfection in ancient Egypt.2
With respect to embalming in ancient Egypt, human bodies were ordinarily treated after passing using the practice of preserving. The word truly implies ‘putting on demulcent or gum’. In fact, it is one of the final stages of the process of protecting the body. The word ‘embalming’ derives from the Latin word (maybe of Persian origin) ‘mumia’ specified by Dioscorides (the first century AD) as a dark mineral found in some places in the desert. This word was afterward used for stuffed objects in Egypt, conceivably from the 26th Dynasty onwards, bituminous materials were generally used to moderate bodies. Mummification is without a doubt the most exceptional method or craftsmanship that was developed in ancient Egypt. Embalmment significantly impacted the traditions and propensities of the ancient Egyptians, and it provided much information on anatomy, chemistry, and numerous practices and industries.
In any country of the world, life is more attractive and more desirable than it was in ancient Egypt… It is no wonder that the Egyptians were portrayed as fanatics of death and dedicated considerable portions of their wealth to devising ways to defeat it. The basic principles of the ancient Egyptian psyche are quite clear in the cries of the dead listed on many funerary paintings in the Central Kingdom, where they ask passers-by to pray on behalf of the deceased: ‘O people who live and live, who love life and hate death, Pass this tomb, because you love life and hate death, so give me what in your hands’ (Lange and Schafer 1902: 30).
Thus, the ancient Egyptians' creative ability driven by the conviction that passing does not essentially end life; or maybe it only speaks to the deterioration of human life, the point at which the soul disavows the body. Starting from 3500 BC, the ancient Egyptians preserved dead bodies of wealthy people totally in different ways. During the New Kingdom period (around 1550–1099 BC), the method included the expulsion and conservation of inner organs. The kidneys and heart which were considered the ‘seat of intelligence and sentiment’ were left in the body. The canopic jars that would house the internal organs were treated with natron and sometimes a hot resin poured over the jars to better preserve them (David 2008).
After the rapidly decomposing organs were removed, ancient Egyptian embalmers dried out the body. According to the Smithsonian Institute, they first encapsulated the body with Natron as an intense drying agent. In addition, the embalmers placed natron beams into the body to absorb moisture. Once the body was dry, the embalmers would wash it and remove the packaging, and here, the packing process begins.
3. The Occurrence of Natron in Ancient Egypt
Natron salt is a chemical salt (Na2CO3) that was used by the Bronze Age communities within the Eastern Mediterranean region for a number of purposes, mostly as a component in the glass industry and as an additive in preparation for mummification (Lucas 1932). Natron salt is composed of the plant remains that grow in salt swamps (called saline plants) or combined with natural sediments. Natron is actually formed from a blend of sodium carbonate and sodium bicarbonate. It is widespread within the Nile Valley in northern Egypt. The Wadi Natrun is a depression in the Libyan Desert, around 40 miles north-west of Cairo. It is roughly 21 miles long and encompasses a chain of lakes with a surface of roughly 76 feet (23 meters) below sea level. When the Nile starts to flow in Cairo from the end of June to September, there is a critical increment within the water supply that enters the valley, and about 30 miles north of the Natron Valley, within the area of Bihars, around 14 miles west of the ruins of the ancient city of Nacratis, there is another sadness but much smaller, somewhat underneath the ocean level, and there are a number of shallow lakes containing natron. Every year in September, water level starts to rise due to the general rise of the surface water level within the delta and the concentration of neighboring channels that work entirely through the Nile Waterway; and in December, the permanent lakes increase in size and other temporary shallower ones are formed. During summer, the area partly dries up, leaving natron in an easily accessible form.
Natural natron varies in colour with the deposit. It may be pure white, dark grey or yellow. It contains soap when mixed with water and was used in the manufacture of soap and mouthwash and as a disinfectant for wounds. The ancient Egyptians used natron as a disinfectant for personal hygiene, and the material was introduced into ceramics, paints, glass-making and preservation of meat.
4. The Use of Natron in Human Mummification
Since Herodotus portrayed the ancient Egyptians' usage of natron salt in embalming process, numerous questions have arisen with respect to the exact strategy of use. To the finest of our information, no scholar in present day times has ever tried to answer these questions. Probably, natron is a natural compound of sodium carbonate and sodium bicarbonate found in Egypt, and it always contains sodium chloride and sodium sulfate as impurities. Since the salt content is often high, even in antiquity there has been some confusion between salt and natron. This disarray is compounded by the reality that numerous analysts at the beginning of this century concluded that the ancient embalmers used salt instead of natron for treating. In his landmark work, Histological studies on Egyptian mummies (1911), Sir Armand Ruffer suggested that the main desiccant in mummification was salt. Dawson (1927) pointed out that ‘For most periods, common salt (blended with a few normal debasements) was the essential preservation figure utilized by Egyptians to treat.’ Basing on early chemical examination of mummies with salt impacts, it was concluded that salt instead of natron was utilized in preserving. Lucas has conclusively demonstrated that the amount of salt is not sufficient to maintain that it was pure salt that was used in embalming, and that the traces could have derived from salt impurities in natron. In support of the position that it was natron and not salt that was used by the embalmers, it should be noted that embalmers' refuse inevitably contains natron and not salt. Unfortunately one of the discoveries of two such caches, H. E. Winlock, uses the words ‘salt’ and ‘natron’ interchangeably, both when referring to his ‘finds’, and to those of Theodore Davis (Brierl and Wade 1996).
5. The Napoleonic Scientific Expedition in Egypt
On July 1, 1798, Napoleon landed in Egypt with 400 ships and 54,000 men and continued to attack the country, similar as he had invaded Italy before. Be that as it may, this Egyptian invasion was different. Together with troopers and mariners, Napoleon brought 150 academics with him – researchers and engineers – who were responsible for capturing not Egyptian soil but Egyptian culture and history. Whereas the military intrusion was eventually a disappointment, the academic attack was fruitful against anyone's wishes. When the mandate to attack Egypt came down, Napoleon saw it as an opportunity to create and establish a nation of Western culture and a territory of the most noteworthy nation in Europe. In addition, he needed to bring a blessing – the gift of most advanced science – to assist the Egyptians in creating their nation, oversee the Nile, raise their agrarian and mechanical yield, raise their standard of living, and stimulate their mental thinking. So he decided to bring with him a corps of researchers, prepared in design, cosmology, normal history, geography, and linguistics.
Meticulous geographical overviews were made, local creatures and plants were examined, minerals were collected and classified, and neighborhood exchanges and industry were scrutinized.3 Most broadly, old Egypt was found – the sanctuaries and tombs of Luxor, Philae, Dendera, and the Valley of the Rulers. Each of these destinations was measured, mapped, and drawn, recording in fastidious detail a pharaonic Egypt never seen by the exterior world.4
The researchers were to be constituted as the Commission of Sciences and Expressions. Napoleon delegated the duty of choosing this commission to three near colleagues: Gaspard Monge, a mathematician, Claude Louis Berthollet, a chemist, and Joseph Fourier, a more youthful mathematician.5 Together, they carefully chosen 150 academics to invite. A few, such as Geoffroy Saint-Hilaire and Deodat de Dolomieu, were set up researchers, but numerous of the engineers were very youthful and came from the recently set up designing schools, the École Polytechnique, the École des Ponts et Chaussées, and the École des Mines. The mission was kept in secret until the final minute – the apparent objective was to attack Britain, not Egypt – and in this way these youthful men indiscriminately travelled by coach and foot in southern France, where they boarded ships for an obscure goal.
In any case, how was the outside world to see what the researchers had found? Luckily, they had stayed in Egypt for six months so the academics had time to make a decision that their discoveries had to be distributed, and they collected and outlined with that point in intellect. After their return to France in 1801, they kept on organizing materials, and at last, in 1809, the first volumes of the Description de l'Égypte were published. Over the years, concluding in 1828, a total of 23 volumes appeared. Three of these volumes were the largest books ever printed, over 43 inches tall. The whole set contained 837 etchings, many of them of phenomenal measure, and they captured the Egyptian culture from each conceivable vantage point. First of all, the volumes of relics, flooding with monoliths, colossi, sanctuaries, sphinxes, and all of antiques were noteworthy. In any case, the volumes of characteristic history, with their crocodiles, asps, lotuses, and palms, were too amazing. Never before had a single country inspired such a monumental encyclopaedia of such depth and splendour. 6
6. Claude Louis Berthollet
The French chemist Claude Louis Berthollet (1748–1822) made numerous unique contributions to both hypothetical and applied chemistry. He was one of the preeminent followers of Lavoisier. He was born on December 9, 1748, within the town of Galloire on Lake Annecy. He attended the College of Turin in Italy, where he graduated in medicine in 1770. In 1772, he moved to Paris to study chemistry.7 In 1778, Berthollet hitched and took a second doctorate in medication at the College of Paris, where his Italian degree was not recognized. By 1780, his published research in chemistry had earned him admission to the Royal Academy of Sciences in Paris, and four years later, he was designated the executive of the Gobelin tapestry artwork works. Here, he con-ducted an extraordinary study of chemistry of dyeing, on which he published an important two-volume work in 1791 (Gillispie 1989; Lemay and Oesper 1946).
In 1785 Berthollet adopted the new system of chemistry based upon the oxidation theory of combustion, developed by the French chemist A. L. Lavoisier. In the same year Berthollet published an important paper on chlorine, describing the bleaching action of this gas in a solution of alkali, which revolutionized the bleaching industry. Unlike his mentor Lavoisier, Berthollet survived the French Revolution unscathed, having served the Revolutionary government as an adviser on technical matters.
In 1798, Napoleon, who was well-informed in science, chose Berthollet to travel with him on the undertaking to Egypt as a scientific advisor. Berthollet need to be a part of the scientific and archaeological institute that Napoleon found out in Cairo, and now it is known that Berthollet perused to start with papers on the topic of chemical affinity, that is, the strengths by which chemical substances are pulled to each other. These papers formed the premise for his two imperative works on hypothetical chemistry, Investigates into the Laws of Chemical affinity (1801) and Essay on Chemical Statics (1803). Berthollet maintained that the masses of drugs included during a chemical response may influence the products which a reaction could also be switched by changing the amounts of the substances. These views drove Berthollet into an extended scientific debate with J. L. Proust about the law of definite proportions. Proust argued that chemical compounds were formed in fixed proportions by weight of their components. Berthollet contended that the proportion by weight of the components seem to vary during a compound concurring to the mass of the reactants from which the compound caused. Proust's view seemed to be vindicated in light of John Dalton's atomic hypothesis, which depends on the law of fixed proportions. In any case, the result was that Berthollet's imperative understanding into the part of responding masses was ignored for quite 40 years. Berthelot's country house at Arcueil, near Paris, became the center which attracted recognized young chemists and physicists to whom he advertised the offices of his private lab. In 1807, this group organized themselves into the Society of Arcueil under Berthollet's supervisoion. His last days were clouded by the suicide of his child in 1810 after the failure of a factory in which he had been greatly interested. Berthollet died at Arcueil on November 6, 1822. 8
7. Berthollet's Findings and the Natron in Egypt
During his mission in Egypt, Berthollet was told about the presence of inexhaustible deposits of sodium carbonate on the shores of the Natron Lakes at the entrance to the desert. Berthollet chose this region to study the possible opportunities for commercial use. In his Memoirs reporting the visit, he wrote that the valley of the Natron Lakes constituted a tremendous research field where nature arranged a monstrous sum of soda. Chemical investigations showed that water from the lakes contained sodium nitrate, sodium carbonate, and a small amount of sodium sulphate which varied from one lake to another. He commented that Lake No. 3, from the six lakes constituting the Natron Valley, is split into two parts, the waters of which have scarcely any communication; the eastern part contains only common salt, while the western had something else than carbonate of soda; so they discovered spontaneous evaporation of marine salt on one side of the lake, and on the opposite – carbonate of soda, each nearly pure. The waters of the eastern segment contained as it were sodium chloride, whereas those of the western area contained almost only sodium carbonate. So, by characteristic vanishing, sodium chloride crystallized on one side of the lake while on its other side there was nearly immaculate sodium carbonate. When the waters contained both sodium chloride and sodium carbonate, at first there crystallized the chloride, taken after by the carbonate, shaping alternating white layers of each salt that were simple to evacuate. Somewhere else, some of these crystals were dissolved by rainwater, which clarified the nearness of natron in water in a closed segment of the lakes.
During summer, the saturated water deposits saline crystals, and when it holds both muriat and carbonate of soda, the former crystallizes first, then the carbonate of soda, in order that there are formed interchange strata of the two salts, which increase annually, given they are not disturbed. Berthollet concluded that sodium carbonate had been and was still being formed by a double decomposition reaction between the saturated brine and the limestone bed of the lakes. The reaction was proposed as early as in 1737 by Henri Louis Duhamel du Monceau (1700–1781) as a conceivable strategy of preparing sodium carbonate (Kiyohisa Fujii 1986; Berthollet and Claude 1799).
Within the region of the Natron Lakes, Berthollet found that the territory covered by sodium carbonate layers did not contain sodium carbonate, but was continuously impregnated with salt; in any case, the landscape in which sodium chloride was decomposing constantly contained an impressive amount of calcium carbonate and was extremely sticky. Sodium carbonate showed up as it were, on the spots where the soil, formed of limestone shake, was splashed with salty water. According to Berthollet, it became clear that calcium carbonate is a substance that leads to the decomposition of sodium nitrate, which it had come into contact with in a hot, sticky environment. The calcium nitrate formed from the decay of sodium nitrate is exceptionally deliquescent and in this way penetrated the profound interior of the terrain. Within the neighborhood where the terrain is especially clayey, the formation of sodium carbonate was particularly favored by the stems of reed, which grew in the region.
The stem favoured the reaction by supporting the efflorescence of carbonate as it was formed. It is interesting that nature was executing the opposite reaction to the one observed in the research laboratory. Berthollet ascribed the reversal of the direction of the reaction due to the difference in experimental conditions between those utilized in the research laboratory and the natural conditions predominant in a particular area. Berthollet had already found that during the extraction of saltpeter from rough nitre rock by dissolution in water, the increasing concentration of saltpeter in solution made the remaining portions more difficult to dissolve, actually despite the fact that water was never immersed with salt. Larger amounts may well be dissolved effectively utilizing fresh water, but each progressive washing yielded a little amount. With the new information gathered at the lakes, Berthollet clarified the reaction by extraordinary amounts of sodium chloride and calcium carbonate and the continuous removal of the products: the deliquescent calcium chloride by drainage below ground. The large quantities in solution of the original substances compared to those of the products maintained a reaction that would not take place by affinities alone. When the salt solution gradually filtered through the pores of the limestone, the generally powerless affinities between these two substances were improved by the combined effects of temperature and gigantic mass of limestone. This led to decay of the salt, guaranteeing a steady generation of sodium carbonate and calcium chloride through double decomposition.
These conclusions led to a renewed interest in Berthollet's earlier suggestion that physical conditions such as temperature, relative concentration, and quantities of reactants affect the nature and direction of affinities in a chemical reaction (as expressed by the laws of chemical action). Berthollet had already examined a paper on this subject at Cairo ‘Recherches sur les Lois de l'Affinité’ which was published by the Mémoires de l'Institut in 1801. This paper was the starting point of his completely new system of chemistry, first briefly outlined in ‘Recherches sur les Lois de l'Affinité’ (1801) and later developed into a comprehensive two-volume Essai de Statique Chimique. In this work, Berthollet developed his main thesis that chemical action is due partly to affinity, which he thought of as similar to gravitational attraction and partly to the masses of the reactants. However, this work also led him to doubt the reality of the constant composition of chemical compounds and was the origin of a lively discussion with Joseph-Louis Proust (1754–1826).
I had the chance to read an interesting paper by Kiyohisa Fujii published in the British Journal for the History of Science (July 1986) under the title ‘The Berthollet-Proust Controversy and Dalton's Chemical Atomic Theory’. Within the transition from history to science, this controversy was a conflict between new and old theories of chemical combination. When Berthollet proposed his point of view, which seemed to open up new theoretical development, even though Proust had followed the traditional theory of elective affinity, his theory gained a new meaning when reinterpreted within Dalton's atomic theory. The debate attracted much attention from contemporary chemists. In France, almost all chemists seemed to support Berthollet's theory. Despite having confessed his doubts about it in 1805, Thenard still supported it in 1821. Gay-Lussac regarded his law of combining volumes of gases as an evidence for Berthollet's doctrine because it was Berthollet himself who maintained that combining proportions were fixed as a consequence of the effect of contraction. In 1828, however, after Berthollet's death, he admitted that Dalton's idea was confirmed by experiments. In 1808, Hassenfratz tried to verify Proust's doctrine by experiments but could not find definite answers. In 1805, Housmann advocated Berthollet for hardly persuasive reasons.
In 1811, based on his own experimental results, Berzelius confirmed Dalton's theory as substantial. However, it appears that he came to treat Berthollet's law of mass action and the law of multiple proportions independently. Hence, in 1813, he accepted that Berthollet's hypothesis of affinity and the laws of chemical proportions might be accommodated with each other. In 1811, Pfaff conducted research that disproved Berthollet's law of mass action.
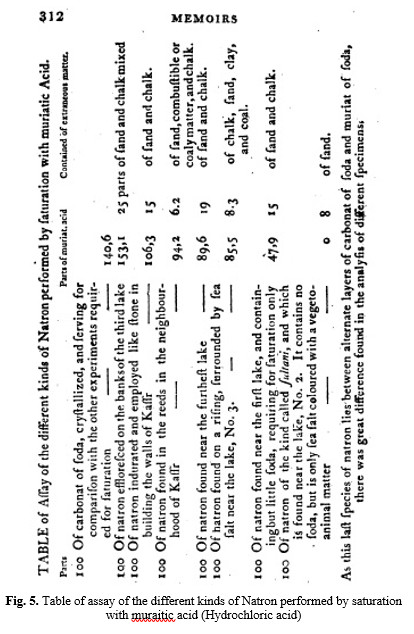
Berthollet published his ‘Observations sur la natron’ in the first volume of the Mémoires sur l’Égypte, and then went on in the next volume to give a more general treatment of the law of chemical affinities. He was planning to present this second paper to the Institute members in August 1799, but instead he was summoned to accompany Napoleon when he secretly fled back to Paris on August 22. However, his early return did allow him to complete his major book, Essai de statique chimique (Paris, 1803). The Essai is recognized as a landmark work that helped found the systematic study of physical chemistry. It will be seen from the Table in the Appendix, that there are some samples of natron which contain much carbonate of soda, and therefore, may be considered as very good soda, but that others are of a much inferior quality, so that in order to establish the use of this article in Europe with confidence, it would be necessary to have an intelligent agent on the spot, to select salt, and to fix different prices according to the quality of natron, which should be determined, not by accidental changes of colour, but actually ascertaining its ratio in carbonate of soda. These works would then become an important branch of the revenues of Egypt.
8. Conclusion
Science is the revelation, reflection, and collection of information that allows us to anticipate the future and ultimately change it. Throughout the history of humanity, scientific progress has been increasingly responsible for technological and societal development. Aristotle's logical revelations and strategies, dating back thousands of years, significantly contributed to the advance of the Western civilization. In modern times, breakthroughs in information theory have led to successive inventions of telegraph, telephone, television and the Internet, making global ideas easier today. Although science is a driving force for improving society, there is no information on how science propels. However, science constantly advances, with every world – past, present and future – leaving an indelible mark on the process of scientific innovation through its research publications, contributing to an ever-growing body of scientific literature.
The French expedition lasted for only three years and three weeks. However, despite this short period of time, scholars and historians agree that this era had two important effects on the long-term development of Egyptian culture: first was associated with the introduction of the principle of equality before the law in Egypt and second was the development of Western culture in Egypt. Napoleon's invasion appeared the primary international communication with Egyptians and may be described as a start of the knowledge inflow at the beginning of the nineteenth century. The Egyptian eyes were opened to new science and humanities brought by the West, enabling Egyptians to independently choose a new ruler (Ead 2019).
While accepting the 1981 Nobel Prize for chemistry, Kenichi Fukui argued that chemistry could make contributions to world peace with the aid of responding to the scarcity of world sources and energy. But his cognizance of chemistry's relation with the world's past, present and future is far from unique. Well over a hundred years before his comments, for example, Karl Marx drew on Justus von Liebig in his analysis of the hazard posed via capitalist exploitation and manufacturing to the global socio-material ‘metabolic system’ (Roberts 2019).
To say that the records of chemistry and global history are related, however, tells us little until we first reflect on consideration on how these two fields of research are – and possibly ought to be – understood. How does it affect our understanding of their interplay, for example, if we equate the records of chemistry with the records of its disciplinary formation and/or international history with the records of globalization? What are the pitfalls of adopting a ‘comparative history’ method to discover happens place when we start to analyse the past by means of following achievements as a substitute for or alongside humans through history? Moving between historic examples and historiographical reflections may advise the ways in which bringing the history of chemistry and international history in nearer contact with each different can bear fruit for both.
In this paper, I have shown a straightforward story of the scientific achievements in the nineteenth century and the subsequent years. At that time, science tended to depend more on the endeavours and efforts of persons, researchers, and among the numerous scientific achievements of the nineteenth century was the appearance of this remarkable chemist. Berthollet succeeded to provide accurate insights into one of the most important assumptions about chemistry. His support during the French campaign in Egypt in 1798 and his visits to the Wadi al-Natrun in Egypt, a place where natron salt is extracted, drove him to create imperative speculations of affinity. Berthollet stayed in Egypt for two years writing about the miracle he had observed and 50 years later this became to be known as the law of mass action.
Finally, I would like to point out that globalization of scientific progress during this period was expected to serve as a starting point to explore the insightful ways in which science had evolved over the course of later centuries, transforming pieces of knowledge into contemporary scientific innovation.
NOTES
1 The Twilight of Napoleon's Egyptian Campaign (1798–1801). Scenes from the Description de l'Égypte https://www.lib.lsu.edu/sites/all/files/sc/exhibits/e-exhibits/egypt/int_one.html.
2 Hamed A. Ead, Ead, H. A. URL: http://www.levity.com/alchemy/islam02.html, http://www.levity.com/alchemy/islam03.html, http://www.levity.com/alchemy/islam25.html.
3 https://napoleon.lindahall.org/learn.shtml.
4 Ibid.
5 https://napoleon.lindahall.org/napoleon_scientist.shtml.
6 https://napoleon.lindahall.org/napoleon_scientist.shtml.
7 https://biography.yourdictionary.com/claude-louis-berthollet.
8 https://biography.yourdictionary.com/claude-louis-berthollet.
REFERENCES
Andreev, A. I., Ilyin, I. V., and Zinkina, J. V. 2015. Approaches and Paradigms in Defining the Essence of Globalization. In Grinin, L. E., Ilyin, I. V., Herrmann, P., and Korotayev, A. V. Globalistics and Globalization Studies (pp. 250–257). Volgograd: Uchitel.
Berthollet, C. 1966 [1799]. Researches into the Laws of Chemical Affinity. Da Capo Press.
Brier, B., and Wade, R. S. 1996. Zeitschrift Fur Agyptische Sprache Und Altertumskunde 124: 89–100. URL: http://journal.plastination.org/archive/jp_vol.11/jp_vol.11_20-21.pdf.
Dawson, W. R. 1927. Making a Mummy. The Journal of Egyptian Archaeology 13 (1/2): 40–49.
David, R. 2008. Egyptian Mummies and Modern Science. Cambridge University Press.
Deacon, R. 2005. Despotic Enlightenment: Rethinking Globalization after Foucault. In Hayden P., et al. (eds.), Confronting Globalization (pp. 34–49). Palgrave Macmillan. https://link.springer.com/chapter/ 10.1057/9780230598829_3
Ead, H. A. 2019. Globalization in Higher Education in Egypt in a Historical Context. Research in Globalization 1 (December): 100003.
Giddens, A. 1990. The Consequences of Modernity. Cambridge: Polity Press.
Gillispie, Ch. C. 1989. Scientific Aspects of the French Egyptian Expedition 1798–1801. Proceedings of the American Philosophical Society 133 (4): 447–474.
Hall, Th. D. 2014. Toward Comparative Globalizations: Globalization in Historical Retrospective and World-Systems Analysis. Journal of Globalization Studies 5 (1): 3–10.
Harvey, D. 2019. 6.1: Reversible Reactions and Chemical Equilibria. In Analytical Chemistry 2.0. DePauw University. URL: https://chem.libretexts.org/Under_Construction/Purgatory/Book%3A_Analytical_Chemistry_2.0_(Harvey)/06_Equilibrium_Chemistry/6.01%3A_Reversible_Reactions_and_Chemical_Equilibria
Johnston, M. R. 1910. The Corsican: A Diary of Napoleon's Life in His Own Words. Boston: Houghton Mifflin.
Josset, P. 1996. Utilisations thérapeutiques du natron dans l'Égypte ancienne et dans le monde gréco-romain. Rev Hist Pharm (Paris) 44 (311): 385–96.
Kiyohisa, F. 1986. The Berthollet-Proust Controversy and Dalton's Chemical Atomic Theory. The British Journal for the History of Science 19 (2): 177–200.
Lange, H. O., and Schäfer, H. 1902. Catalogue Général: Grab- und Denksteine des Mittleren Reichs im Museum von Kairo. Vol. I.
Lemay, P., and Oesper, R. E. 1946. Claude Louis Berthollet (1748–1822). Journal of Chemical Education. 23 (4): 158.
Lucas, A. 1932. The Occurrence of Natron in Ancient Egypt. Journal of Egyptian Archaeology 18 (1): 62–66.
Memoirs Related To Egypt. 1800. Written by Learned and Scientific Men who accompa-nied the French Expedition, printed by T. Gillet and others, London.
Mubashar, H. 2011. The Concept of Globalization and how this has impacted on Contemporary Muslim Understanding of Ummah. Journal of Globalization Studies 2 (2): 145–159.
Quibell, J. E. 1908. The Tomb of Yuaa andThuiu. Cat. Gen. du Musee du Caire VI: 75–77.
Roberts, L. 2019. What the History of Chemistry and Global History Can Offer Each Other? The 12th International Conference on the History of Chemistry, Maastrich.
Weller, S. W. 1999. Napoleon Bonaparte, French scientists, Chemical Equilibrium, and Mass Action. Bull. Hist. Chem. 24: 64.